From Vision to Reality: The Industrial Solution of the Concept Presented at VivaTech 2024
At Praesens Care, we are proud to be the technology integrator for the U1stVision project, showcased as an innovative concept at the VivaTech Show. With our extensive experience in designing, building, and deploying mobile health infrastructure, we are well-equipped to turn this groundbreaking concept into a fully realized product that can transform healthcare delivery and improve lives globally.


U1stVision addresses critical challenges in healthcare, particularly in underserved regions and medical deserts, by providing patients with access to care anytime, anywhere. While the concept presented at VivaTech is highly promising, Praesens Care is ready to bring this vision to life as a scalable, practical solution. We have the expertise to adapt this mobile healthcare platform into a fully operational system that can be deployed at scale.
This platform integrates cutting-edge technologies like artificial intelligence, enabling fast, accurate diagnoses, while offering essential services such as preventive care, diagnostics, and disease monitoring. The flexible, modular design of U1stVision ensures that healthcare professionals can customize the platform to meet the specific needs of their practice, making it an invaluable tool for both public and private sector providers.
At Praesens Care, we believe in the power of technology to drive meaningful change in healthcare. By translating U1stVision from concept into a real-world solution, we are poised to make a significant impact on healthcare access, reduce patient wait times, and ease the burden on healthcare systems, ultimately improving outcomes for patients worldwide.
We’re not just imagining the future of healthcare—we are building it.
Presenting the concept at MedinTechs between 10-11th March 2025
![]()
Introducing the Pathogen Diagnostics Readiness Index (PDxRI)
To address the gaps in diagnostic preparedness, the Pathogen Diagnostics Readiness Index (PDxRI) was developed as a comprehensive tool to assess the global diagnostic landscape. Created by FIND and OneTandem, the PDxRI evaluates the availability of diagnostic tools for a range of pathogens known to cause outbreaks, epidemics, and pandemics.
The first iteration of the PDxRI covers 21 key pathogens, including familiar names like SARS-CoV-2, Ebola, Mpox, and others. It provides insights into which diagnostic tools are suitable for various healthcare settings, their regulatory status, and the availability of target product profiles (TPPs).
As an example SARS-CoV-2 is scoring high and Salmonella Enterica low.
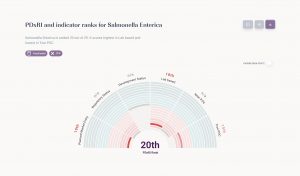
The tool aims to:
The PDxRI plays an invaluable role in improving diagnostic access and epidemic preparedness by offering evidence-based insights that guide decision-making. It identifies where diagnostic tools are lacking and helps public health officials, policymakers, researchers, and diagnostic developers prioritize resources, ensuring that health systems are better equipped to handle future outbreaks.
Praesens Care’s Commitment to Diagnostic Access and Epidemic Preparedness
The Pathogen Diagnostics Readiness Index (PDxRI) offers valuable insights into gaps in the availability of diagnostic products for critical pathogens. At Praesens Care, we align our mission with these insights as we are dedicated to improving diagnostic access and strengthening epidemic preparedness by designing, building, and deploying mobile medical laboratories equipped with essential diagnostic tools.
Our mobile labs are designed and equipped to test for all 21 pathogens on the PDxRI, addressing the gaps identified in diagnostic products, particularly between laboratory-based tests and point-of-care (POC) tests. While many diagnostics score well in the "lab-based" category, they often fall short in true POC settings, where rapid, accessible testing is crucial. With our mobile labs, we can also bring these laboratory-based diagnostics to the point of care, effectively bridging this significant gap.
This allows us to offer high-quality, lab-grade testing for all of the 21 pathogens in a decentralized, field-ready manner, independent if they score high or low on POC. By providing real-time, on-site testing in underserved or remote areas, we ensure faster detection, intervention, and containment of diseases. This integration of lab-based diagnostics into POC environments helps fill a critical gap in epidemic preparedness, bringing essential tools to regions where healthcare infrastructure is limited.
The PDxRI serves as a key framework that informs our approach. By revealing which pathogens are in greatest need of diagnostics and identifying gaps in the diagnostic landscape, the PDxRI enables us to plan our deployments effectively. By matching our deployments with the needs revealed by the PDxRI, we enhance epidemic preparedness by delivering the necessary diagnostics to communities that would otherwise lack access, ultimately strengthening health systems and preventing the uncontrolled spread of infectious diseases.
Praesens Care remains committed to working alongside health authorities and diagnostic experts to enhance diagnostic access and improve epidemic preparedness worldwide. Our innovative mobile solutions help ensure that even the hardest-to-reach locations have access to the diagnostics they need to respond quickly and effectively to emerging health threats.
https://www.finddx.org/data-and-impact/dashboards/diagnostic-readiness-index/#/
In late 2023, the WHO unveiled its 4th Edition of the EDL, reaffirming its dedication to updating this guide with evidence-based choices for in vitro diagnostics (IVD). The latest list includes several new additions and revisions, with three notable highlights:
The EDL4 introduces three tests for Hepatitis E Virus, marking a significant update that includes a rapid diagnostic tool for enhanced diagnosis and surveillance of HEV infections. Given the global prevalence of Hepatitis E and its potentially severe outcomes, these new entries aim to improve outbreak management and assist governments in effectively addressing reported and under-reported cases.
EDL4 has expanded to include personal use glucose monitoring devices, complementing existing medical recommendations for diabetes management. With diabetes being a leading global health concern responsible for 1.5 million deaths annually, the inclusion of personal glucose monitoring devices is set to improve disease management, enabling better outcomes through consistent monitoring.
Reflecting a holistic approach, the EDL4 extends its scope to encompass tests for endocrine disorders, reproductive, maternal, and newborn health, as well as cardiovascular health. Among the new additions are tests for parathyroid hormone, 17-hydroxyprogesterone, Kleihauer-Betke acid-elution, and high-sensitivity troponin I and T, catering to a wide array of health conditions.
Empowering Countries in Diagnostics Planning
The EDL offers a valuable policy framework rather than being prescriptive. It supports countries in improving access to IVDs by providing evidence-based recommendations for their national Essential Diagnostics Lists. Governments can use the EDL to enhance in vitro diagnostics testing services, leading to greater diagnostic access and improved patient outcomes.
Global Collaboration and Support
Aligned with the recent WHA 76.5 resolution on strengthening diagnostics capacity, the release of the EDL4 encourages Member States to develop national diagnostics strategies and essential diagnostics lists based on the WHO model. WHO actively assists countries through webinars, workshops, and direct support, aiding them in creating and implementing EDLs.
Conclusion
The 2023 Essential Diagnostics List marks a significant advancement in global healthcare, providing a roadmap for countries to enhance diagnostic capabilities and improve patient care. By embracing innovation and addressing a variety of health challenges, the EDL reaffirms WHO's dedication to fostering a healthier world through informed, evidence-based diagnostics.
Praesens Care Commitment
Praesens Care continues to leverage this crucial framework to align and expand our BIONEAR service offering, bridging the last mile and enhancing diagnostic access, always tailored to the local health landscape. We are deeply committed to our mission, aiming to:
Through our mobile laboratory, we adeptly navigate challenges even in the most resource-limited settings. Our unwavering dedication to strengthening health systems brings us closer to patients and essential points of need.

For detailed insights into the 2023 WHO EDL decisions, visit the link https://www.who.int/publications/i/item/9789240081093.
The importance of timely laboratory diagnosis
Timely laboratory diagnosis is pivotal in healthcare, particularly in resource-constrained regions, where disease burdens are pronounced. Challenges like inaccessibility, unaffordability, and lack of infrastructure have hindered timely diagnosis, making laboratory diagnostics a critical weak link in the care continuum for many prevalent conditions.
The Kenya Harmonized Health Facility Assessment (KHFA) of 2018-2019 revealed that the mean availability of expected diagnostic tests in Kenya stood at 56%, with urban health facilities boasting 69% availability, compared to 50% in rural areas.
Effective diagnostic tests not only limit healthcare costs but also mitigate trial-and-error treatments and overprescription. They expedite treatment initiation, reducing hospital stays. Multiplex testing, as offered by BIONEAR, provides a comprehensive diagnostic profile, potentially reducing the number of tests required, especially in cases with similar causative agents or during urgent outbreaks.
Strengthening the Community Health Systems by combining quality diagnostics in a mobile laboratory with existing health infrastructure – a pilot study
In collaboration with the Ministry of Health, Foundation for New and Innovative Diagnostics, Praesens Care, and JKUAT, a mobile laboratory (BIONEAR) was deployed in Mombasa through the County Health Management Team to bolster community health systems. This strategic initiative targeted priority pathogens prevalent in the county, leveraging existing healthcare infrastructure.
Mombasa County was chosen as the pilot site due to its specific pathogen profile, diagnostic needs, and geographical and social features. Over a 4-month period, the mobile laboratory was routinely deployed to conduct four categories of tests—Molecular, Biochemical, Serological, and Hematological—in support of 13 public county health facilities spanning six sub-counties. Additionally, BIONEAR actively participated in eight county-sponsored medical community outreaches.
Throughout these efforts, a total of 2395 diagnostic tests were conducted, highlighting the effectiveness of integrating quality diagnostics within the community health framework.
The pilot study had 3 important objectives:
Support in public Health Emergencies and Outbreak Control
The BIONEAR contributed significantly to augmenting disease surveillance activities of the County Department of Health.
Notably, during a diarrhea outbreak in children and a cholera emergency in Mombasa County, the multiplex testing capabilities of BIONEAR proved invaluable. With onsite testing facilitated by the mobile laboratory, the short turnaround time enabled swift implementation of containment measures by the County Health Department. This collaborative partnership not only expedited outbreak response but also significantly reduced both time and costs associated with sample analysis, offering a rapid and cost-effective solution to emergent health threats.
Capacity strengthening
The personnel involved in this pilot study experienced skill acquisition and knowledge enhancement through the utilization of high quality diagnostic equipment provided by BIONEAR. Consequently, the deployment of BIONEAR served not only as a diagnostic tool but also as a training and capacity-building platform, facilitating knowledge transfer and skill development among laboratory staff.
Turnaround time
The introduction of BIONEAR expedited turnaround time (TAT) for clinicians, enabling prompt prescription of appropriate medications or subsequent courses of action in patient management. This reduction in TAT not only alleviates the burden of travel for patients, thereby saving time and costs, but also minimizes the risk of infectious disease transmission and reduces the likelihood of patient loss to follow-up.
This partnership resulted in:
“I am glad to note that the laboratory played a significant role in providing support for surveillance and public health emergency services in Mombasa County.” Dr. Swabaha Ahmed Omar, CEC Health, Mombasa County
Praesens Care commitment
At Praesens Care, we are dedicated to enhancing local capacity and positively impacting patient health outcomes. Our mobile medical laboratory, BIONEAR, is poised to play a pivotal role in addressing the accessibility gap in diagnostics. By offering advanced diagnostics paired with a reduction in turnaround time, travel duration, and patient loss to follow-up, BIONEAR can significantly influence prevention, surveillance, diagnosis, and monitoring efforts.
We are committed to providing an adaptable platform that can cater to diverse local needs and objectives. Whether it's surveillance of non-communicable diseases or rapid deployment during disease outbreaks at the county or national level, BIONEAR stands ready to meet the challenge.
Our approach emphasizes the fusion of local empowerment with high-quality diagnostics and care. We believe this combination is instrumental in achieving more efficient outcomes than those traditionally offered by public facilities. With Praesens Care and BIONEAR, communities can access timely, accurate, and tailored healthcare solutions, ultimately improving overall health and well-being.
Recently (2023), the World Bank and IFC (International Finance Corporation) launched a report on how PPPs (Private-Public-Partnerships) can help improve access to affordable diagnostic services in emerging markets.
In the report they share feedback from interviews with stakeholders of PPPS around the globe and lessons learned from case studies.
The essential role of clinical diagnostics
They restate the essential role of Clinical diagnostics:
However global threats like the COVID-pandemic have shown there is still a widespread scarcity of diagnostic capacity and capability in many low and middle-income countries (LMICs) mainly due to deficiencies in
PPPs could be one of the approaches to support improvement in access to contemporary diagnostics
While highlighting the benefits of PPPs they also describe some risk mitigations to ensure successful Private-public partnerships.
Benefits of PPPs
Risk Mitigations for successful PPPs
The also share 3 success stories of PPPs around the globe, where PPPs created a better access to public health facilities by improving the availability of laboratory tests, high complexity tests and vital health services.
Praesens Care commitment
Praesens Care also believes in the power of innovative private public partnerships to enhance access to affordable diagnostics and care. We therefore opt for operating in a decentralized manner, deploying mobile platforms out of local hubs, staffed by local teams, to optimize the scarce qualified human resources and equipment, but with the support and operational expertise of the global Praesens team.
By offering support services including (tele)maintenance, comprehensive training packages, supply chain of reagents and spare parts, etc., we align the necessary components for optimal utilization of the assets and hence the sustainability of the operational model.
Furthermore, quality assurance systems, such as the certification of professionals and the accreditation of services also fall behind industry best practices in some countries. As such, the BIONEAR that is adherent to and accreditable for ISO15189 – Medical Labs, directly contributes to complying with the highest regulatory requirements and standards.
The combination of local empowerment and high quality diagnostics and care, are crucial for achieving more efficient outcomes than those that have historically been provided by public facilities.
Read the full article: https://www.ifc.org/content/dam/ifc/doc/2023/2023-PPP-Insights-Health.pdf
linked in: https://www.linkedin.com/feed/update/urn:li:activity:7120752325786234880
On the occasion of the Belgian National Day on the 21st of July, Praesens Care is proud to witness the BIONEAR took part in the Military Parade in Brussels.
Among the new vehicles demonstrated by the Belgian Army's Medical Component, this variant acquired by the Belgian Defense, drew the attention of both connoisseurs and the general public. A total order of three fully-equipped vehicles was placed, two of which were donated to Ukrainian Armed Forces in an effort to offer medical support to patients, hit by the war.
This project perfectly fits in an "Equip & Train" approach to ensure the end-users, beyond receiving state-of-the-art capacities can count on comprehensive training, maintenance and support services offered by Praesens Care.
Emmanuel Vidal, CEO of Praesens Care: "In the toughest and most demanding conditions, an army wants to be able to rely on its equipment and ensure the personnel's safety. We are, therefore, proud that the Belgian army has chosen the unrivaled reliability and qualities of our BIONEAR. This proves the versatility of our vehicles provides an excellent integrated biomedical lab solution for all military applications on national territory and beyond."
In this edition on “Bioresponse”, Praesens Care presents its 3rd generation BIONEAR®, its commercially available, fully autonomous, and connected molecular biology mobile lab, with unique genomic capacities on board.
Praesens Care is proud to have developed with its partners a truly European sovereign solution (mostly the fruit of a French-Belgian collaboration) that addresses a longtime identified gap in European capacities for NATO.
It was a pleasure to be participating in the Finale in Skopje on June 28th.
You can rewatch the live broadcast through the following link: https://www.youtube.com/watch?v=GgLeLxg_mOE (Praesens Care pitch starting at 02:35:00)
In October 2021, The Lancet Commission on Diagnostics launched a publication to put the spotlight on the current status of the diagnostic landscape. It highlighted key gaps in global diagnostics and articulated a series of recommendations to act as a roadmap for more equitable access to diagnostics.
The scope of the publication was remarkably broad and the Commission identified fundamental flaws at all levels of the diagnostic provision pathway: infrastructure, workforce, quality, safety, financing, visibility, and policy. Lack of prioritization and scarce allocation of resources were underlined as overarching problems, leading to weak and undersupplied health systems.
Overall, the report provides evidence of poor access to accurate diagnostics for almost half of the global population, especially at the level of primary health care (the so-called diagnostic “last mile”). Diagnosis is shown to be the biggest gap in the cascade of care (comprising screening, diagnosis, treatment, and control), with adverse consequences for the burden of disease.
The publication comes in a timely manner, now that the COVID-19 pandemic has underscored how crucial diagnostics are for early detection and response to emerging infectious diseases. However, the power of diagnostics reaches far beyond epidemic preparedness and control. While initial diagnosis serves as entry point to the care cascade, diagnostics have further implications that range from guidance to therapy and prognosis, to monitoring of disease progression and response to therapy.
Among the Commission’s recommendations are the implementation of point-of-care testing at the primary health care level and the continued fostering of innovation, especially in low-and-middle-income countries (LMICs). Reducing the “last mile” diagnostic gap will increase the uptake of health services and improve health outcomes. In fact, the Commission’s analysis has shown that reducing the diagnostic gap for six priority conditions alone (diabetes, hypertension, HIV and tuberculosis in the overall population, and hepatitis B virus infection and syphilis for pregnant women) can avoid over one million premature deaths annualy in LMICs.
Praesens Care is actively endorsing the ten recommendations that are put forward in this publication to help transform access to diagnostics. Beyond offering the above tracer tests on-board of our bioNears, our assay menu has also been largely inspired by the National Essential Diagnostic Lists to best address unmet diagnostic needs in the countries where we operate.
Our spirit embodies a strong will to ensure sustained access to quality diagnostics for everyone. We are committed to a holistic health care delivery model, providing diagnostic capacity that is integrated in the care cascade, embedded in the local health systems, and as close as possible to the patients. We embrace decentralization as a vehicle for change towards a world where health systems are resilient, better prepared, and more equitable.